Research Interest
- Electrical and optical properties of chalcogenide glasses
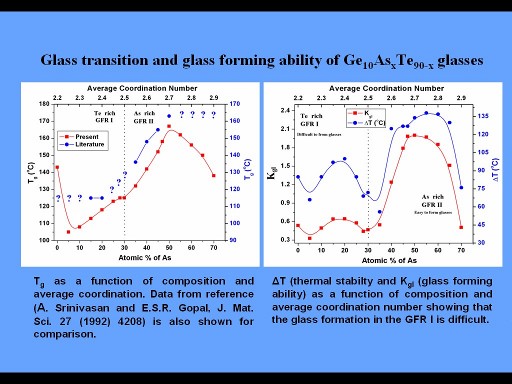
Chalcogenide glasses are formed from Group VI elements S, Se and Te combined with Group I, III, IV, V, VII group elements. They are generally prepared in vacuumed silica ampoules by melt quenching method. Many times this results in a heterogeneous composition because of the poor thermal conductivity of silica. Also the dimension of the quenched glass is limited by the diameter of the silica tube. We are interested in preparing these glasses by alternate methods like mechanical milling, spin coating and solution methods. XRD, MDSC, Raman, infrared, XPS, NMR methods will be used to characterise these glasses.
Structural relaxation, electrical conductivity, electrically driven phase change property, optically induced transitions, crystallisation kinetics, glass forming ability, rigidity percolation and intermediate phases will be studied. These properties will be used to arrive chalcogenide glass systems with specific composition range useful for thermoelectric energy conversion, infrared detection and toxic gas sensing applications.
- Synthesis of ultra-hard carbon nitride (C3N4)
Carbon nitride is predicted to be an ultrahard material with low friction coefficient and high wear resistance which is very unique. The N/C ratio should be 1.33 (43 % C and 57 % of N). Attempts in increasing the N content increases the graphitic C3N4 phase which results in low hardness. We are successful in preparing the C3N4 with more than 53 to 55 % of N by simple CVD method. Constant efforts in optimizing the preparation conditions are being made to achieve the phase pure C3N4 with high hardness and wear resistance.
- Pressure induced phase transitions
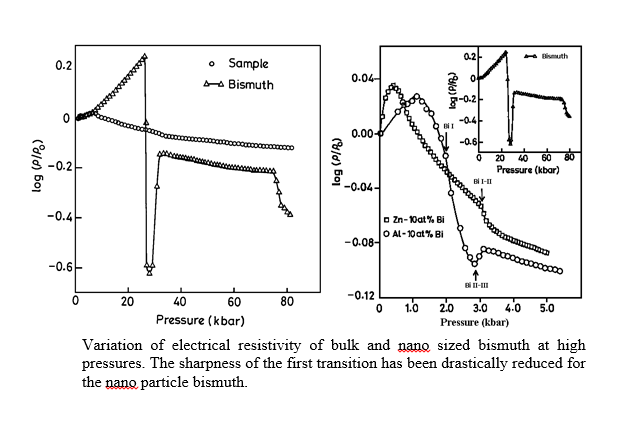
Structural and phase transitions of solids at high pressures and high temperatures (up to 350 C) and low temperatures (down to liquid nitrogen temperatures) have been studied by monitoring the electrical resistivity in a Bridgman Opposed Anvil setup. For example, semiconductor - metal transition, glass - crystal transition and shift of glass transition with pressure are being studied.
- The figure shows the effect of high pressure on the glass transition temperature(Tg). Usually Tg increases with pressure (according to the thermodynamic models like Free Volume and Entropy). In As40Te60 and Ge20Te80 glasses a decrease in Tg (negative dTg/dP) has been observed. It has been suggested that when the thermal expansion of the glass is negative, dTg/dP can be negative. High temperature neutron diffraction experiments show a negative thermal expansion for most of the Te based chalcogenic glasses in their liquid and supercooled liquid states. The decrease in Tg with pressure can also be understood from Tg-Eg-C model which finds linear relation between glass transition and optical band gap when grouped according to the connectivity. Application of high pressure decreases the optical band gap. Hence, the decrease of Tg with the increase of pressure can also be explained with this relation.
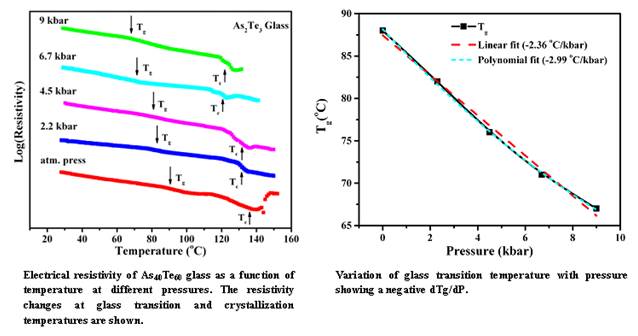
K. Ramesh, "Pressure Dependence of Glass Transition in As2Te3 Glass", J. Phys. Chem B 118 (2014) 8848-8853
-
The figure shows the electrical switching in Se doped Ge-Sb-Te (GST) glasses (which are used in rewritable CDs, DVDs and Blu-ray disks). At the time of switching the amorphous film between the electrodes undergo phase change between the amorphous and the metastable crystalline NaCl structure due to Joule heating. The amorphous to NaCl structural phase transition occurs at about 1500C. GST also crystallises to a stable hexagonal structure at about 250oC. The general understanding is that the presence of metastable NaCl structure facilitates the switching with ease and fast because of the structural similarities between amorphous and metastable phases. Our study shows that the Se doped GST undergoes a direct transition to stable hexagonal phase with comparable switching voltages. This indicates that the GST based alloys need not necessarily transform to the metastable NaCl structure at the time of switching.
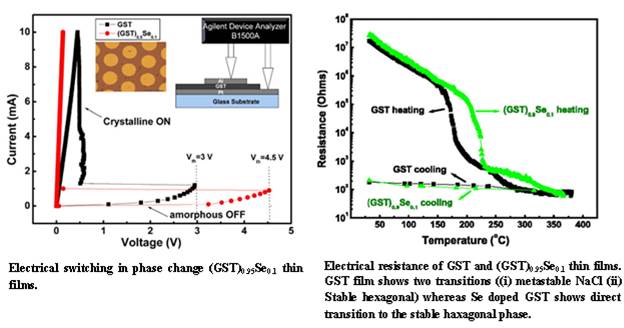
E.M. Vinod, K. Ramesh, K. S. Sangunni, "Structural transition and enhanced phase transition properties of Se doped Ge2Sb2Te5 alloys", Scientific Reports 5, Article number:8050 doi:10.1038/srep08050